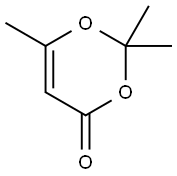
![Early Amidation Approach to 3-[(4-Amido)pyrrol-2-yl]-2-indolinones | The Journal of Organic Chemistry Early Amidation Approach to 3-[(4-Amido)pyrrol-2-yl]-2-indolinones | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo034304q/asset/images/large/jo034304qn00001.jpeg)
Early Amidation Approach to 3-[(4-Amido)pyrrol-2-yl]-2-indolinones | The Journal of Organic Chemistry

2,2,6-Trimethyl-1,3-dioxin-4-one, cont. up to ca 6% acetone, Thermo Scientific Chemicals | Fisher Scientific

Effect of ketene additive and Si/Al ratio on the reaction of methanol over HZSM‐5 catalysts - Hassanpour - 2018 - Applied Organometallic Chemistry - Wiley Online Library

EP0614904A1 - Method for the preparation of Isopropylidene derivatives of saccharides - Google Patents

Diketene a Privileged Synthon in the Synthesis of Heterocycles. Part 2: Six-Membered Ring Heterocycles - ScienceDirect

End group modification of polyamide-6 in supercritical and subcritical fluids: Part 3: Amine end group modification with diketene and diketene acetone adduct in CO2 - ScienceDirect


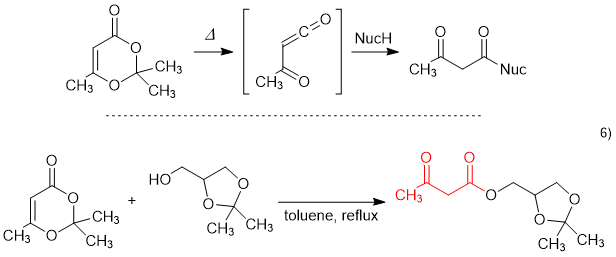
![2,2,6-trimethyl-m-dioxin-4-one - Optional[1H NMR] - Spectrum - SpectraBase 2,2,6-trimethyl-m-dioxin-4-one - Optional[1H NMR] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/9pPIMRR7F5H/structure.png?h=300&w=382)




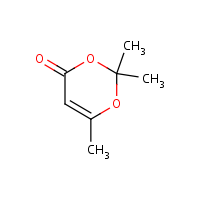




![2,2,6-trimethyl-m-dioxin-4-one - Optional[1H NMR] - Spectrum - SpectraBase 2,2,6-trimethyl-m-dioxin-4-one - Optional[1H NMR] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/9pPIMRR7F5H/partial.png?h=214.875&ph=true&w=382)